Trust the ONLY hangover pill to be fully tested

Good Morning
Morning Recovery
All Other Products
Why it matters
Grant funded research
Ensures credibility, financial backing.
IRB Approved
Protects participant safety and ethics.
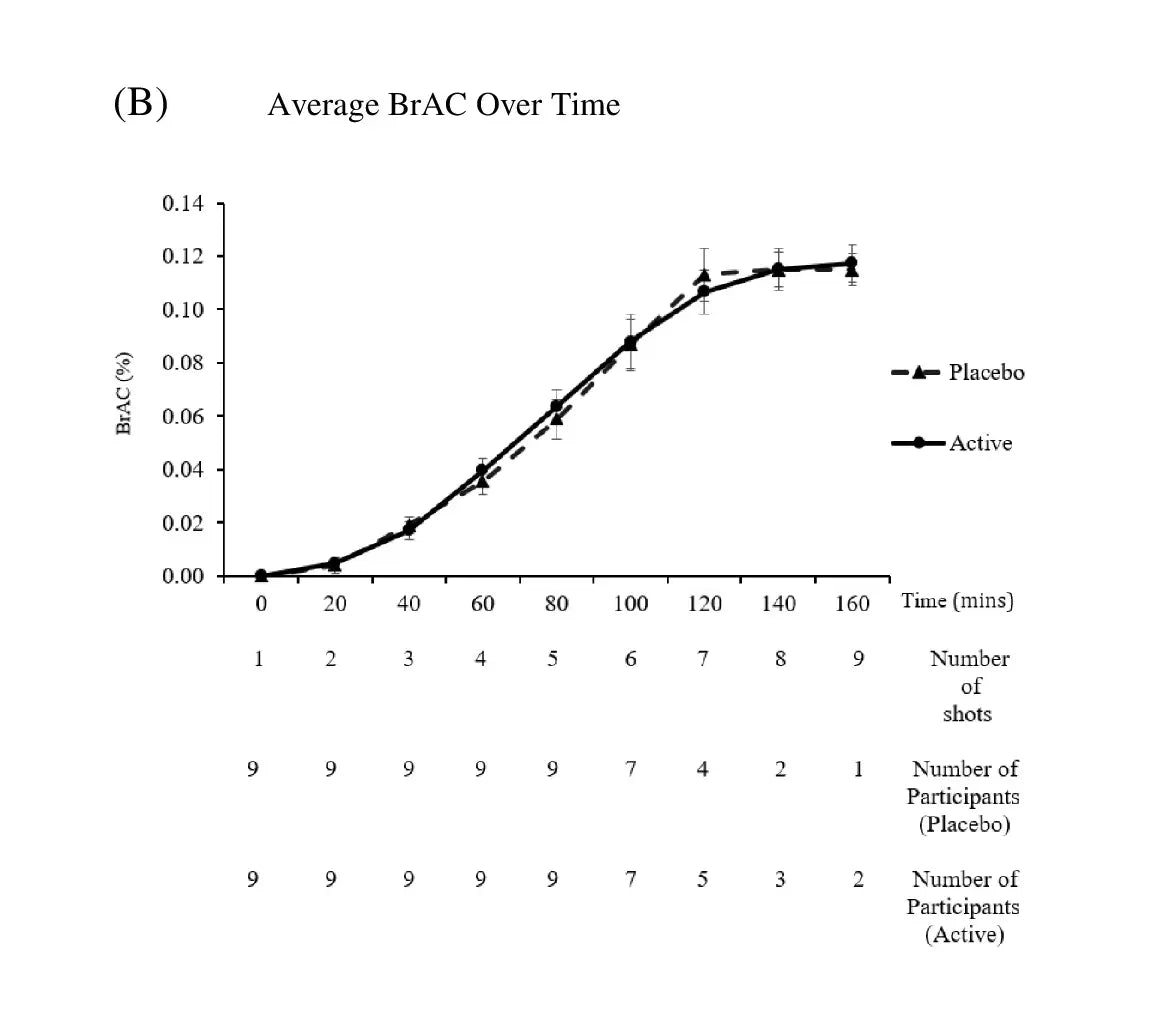
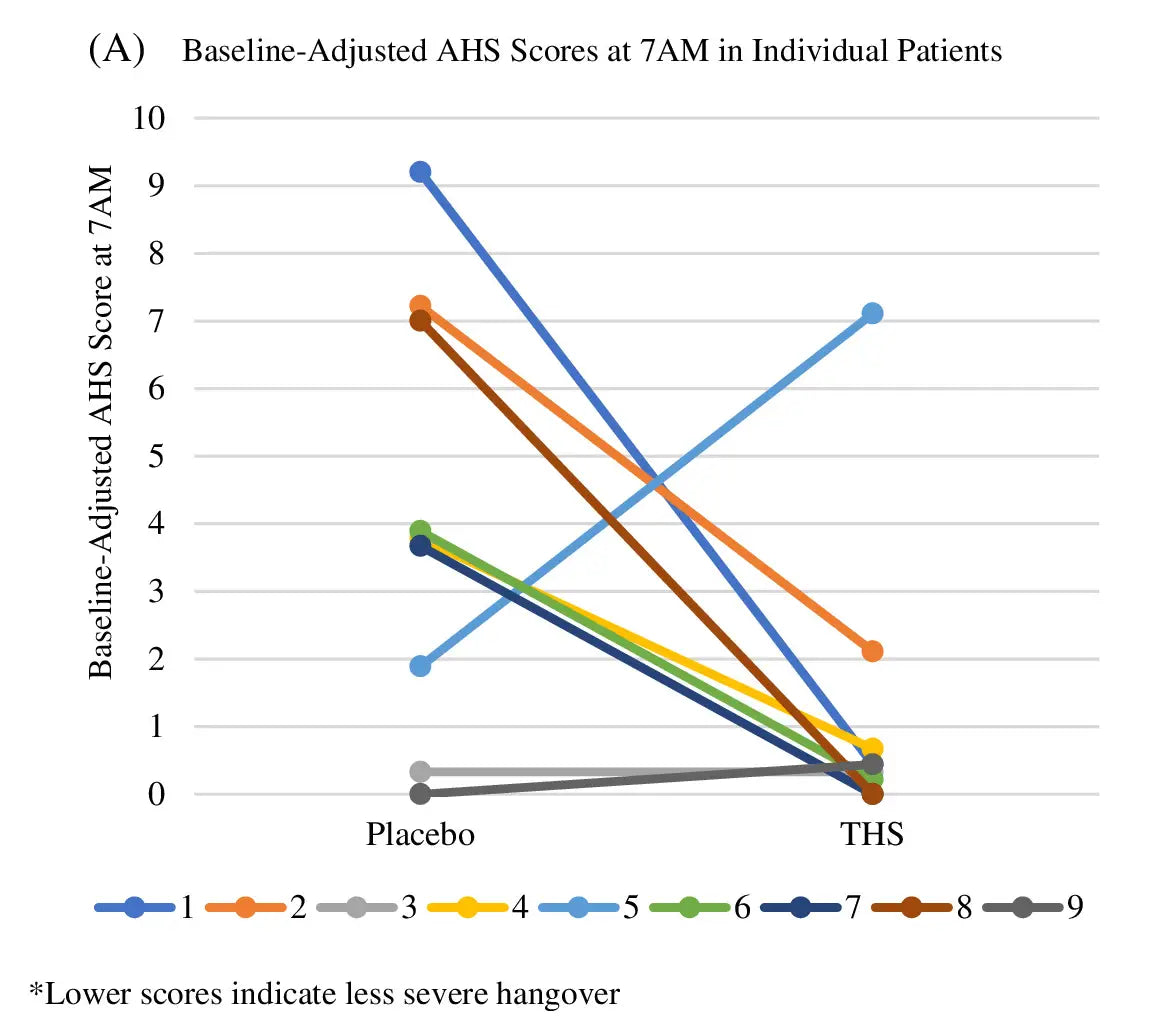
Double blind, Placebo controlled
Eliminates bias, ensures objectivity.
Independent, Randomized
Enhances validity, reduces confounding.
Cross-over clinical trial
Increases reliability, reduces variability.
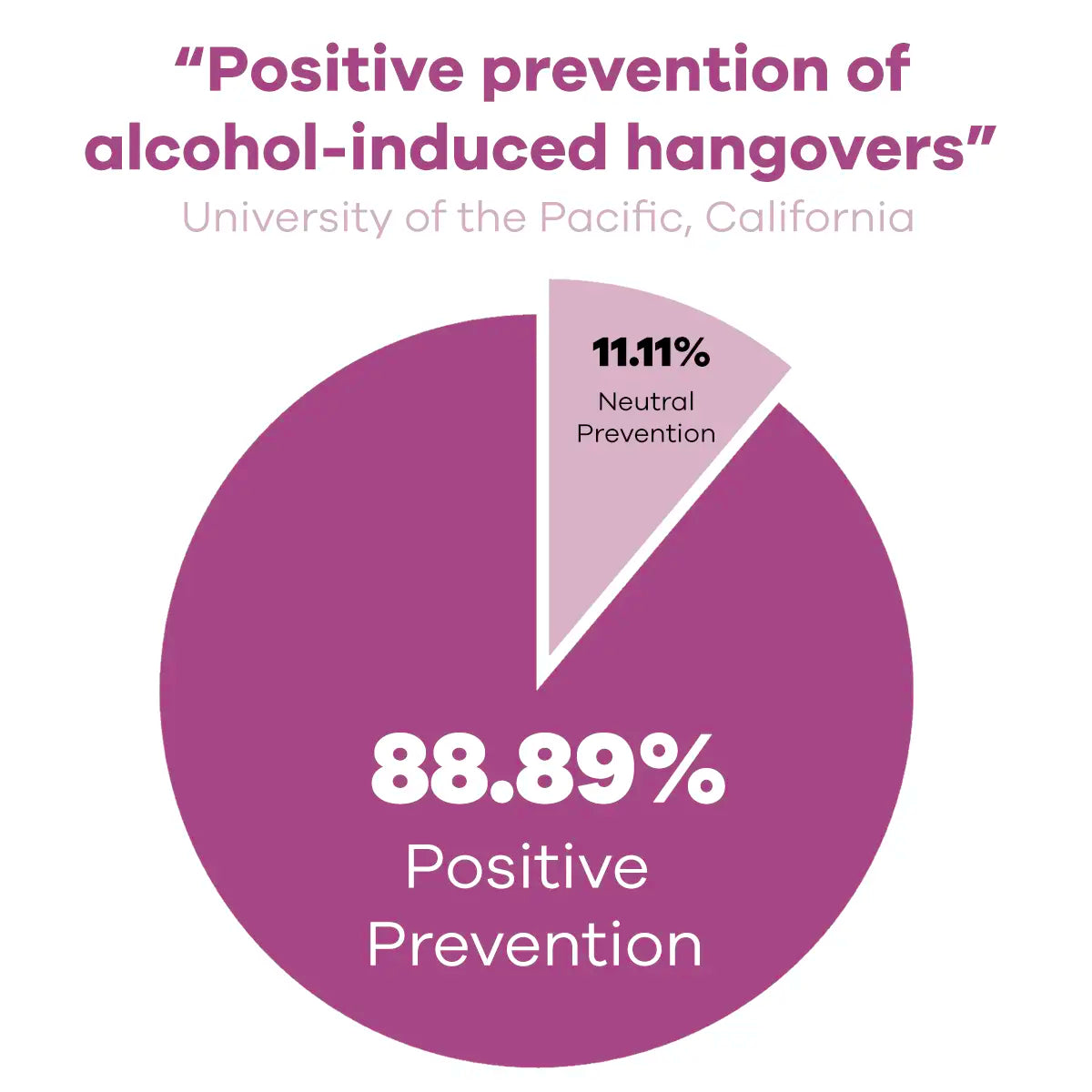
Peer-Reviewed
Validates quality, expert scrutiny.
Published in Medical Journals
Widens credibility, professional dissemination.
Pharmacy Level Grade Clinical Research
High standard, rigorous testing.